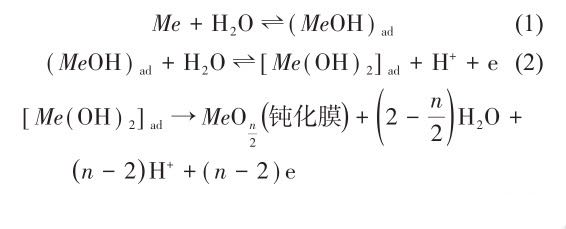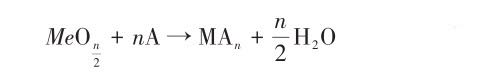The research on the corrosion resistance of high-entropy alloy coatings in recent years is summarized, mainly from the preparation process, alloy composition and process parameters on the corrosion resistance of high-entropy alloys, and the existence of corrosion-resistant high-entropy alloy coatings Suggestions on the issues and research priorities. The authors of the above work are Jia Chuntang and Sha Minghong, School of Materials and Metallurgy, Liaoning University of Science and Technology.
Insufficient high temperature stability of most traditional alloys will reduce their mechanical properties and corrosion resistance, thus limiting their application in extreme and highly sensitive engineering environments. In 2004, Yeh et al. broke through the traditional alloy design concept and proposed the concept of high-entropy alloys (HEAs). Initially, high-entropy alloys were defined as containing more than 5 main elements and the content of each main element was 5%~35% ( Atomic fraction) between a new type of alloy. Due to the effect of high mixing entropy, high-entropy alloys tend to form simple solid solution structures such as face-centered cubic (FCC), body-centered cubic (BCC) or close packed hexagonal (HCP) structures, rather than complex intermetallic compounds. The special composition and structure make the high-entropy alloy possess heat resistance, wear resistance, corrosion resistance, and good magnetic properties. Therefore, high-entropy alloys are expected to become candidate materials in some extreme and highly sensitive engineering environments such as nuclear power, turbine engines, and aerospace.
Because it contains many expensive metals (such as Nb, W, Cr, V, Ni, Ti, etc.), the cost of high-entropy alloys may be higher than most conventional alloys, and surface coatings can solve this problem. In recent years, researchers have successfully prepared high-entropy alloy coatings through processes such as laser cladding, electric spark deposition, electrochemical deposition, electron beam evaporation, and magnetron sputtering. By using high-entropy alloy coatings, a reasonable combination of cost and performance can be achieved.
Corrosion resistance mechanism of high-entropy alloy coating
Figure 1 is the corrosion behavior diagram of high-entropy alloy coating, bulk high-entropy alloy and stainless steel in 3.5% (mass fraction) NaCl solution. As can be seen from the figure, compared to the bulk high-entropy alloy, the high-entropy alloy coating has a lower current density (I corr) and a higher corrosion potential (E corr), and the corrosion resistance is similar to that of stainless steel . Figure 2 shows the pitting behavior of high-entropy alloy coatings, bulk high-entropy alloys and stainless steel in 3.5% (mass fraction) NaCl. It can be seen that the high-entropy alloy coating has lower corrosion resistance and higher pitting resistance potential (E pit). Compared with stainless steel and bulk high-entropy alloy, high-entropy alloy coating shows better resistance to pitting Eclipse performance.

Figure 1 Corrosion behavior of high-entropy alloy coating, bulk high-entropy alloy and stainless steel in 3.5% NaCl solution
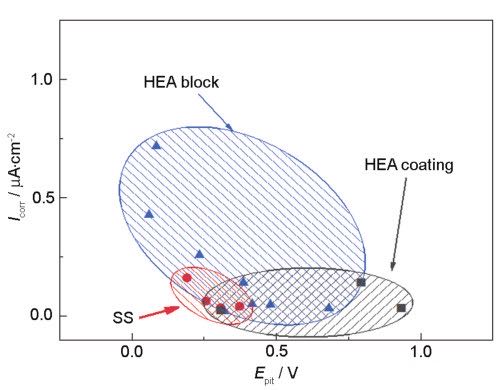
Figure 2 Pitting corrosion behavior of high-entropy alloy coating, bulk high-entropy alloy and stainless steel in 3.5% NaCl solution
The corrosion resistance mechanism of high-entropy alloys can be summarized as the following 3 points:
(1) Due to the high-entropy effect, high-entropy alloys are easier to form a single solid solution phase or amorphous phase than traditional alloys. As we all know, the single phase composition, the more uniform the composition. The formation of a single solid solution or amorphous can reduce the effect of galvanic corrosion and the number of micro-batteries, thereby improving corrosion resistance.
(2) The addition of elements such as Cr, Ni, Cu, Ti and Mo can produce a passivation film on the coating surface. In oxidizing acids such as nitric acid and concentrated sulfuric acid, these elements are easily oxidized to form a dense oxide film, such as Al2O3, CrO3, Cr2O3 film, etc., thereby reducing the corrosion rate. In an alkaline solution, the surface of the alloy added with corrosion-resistant elements is likely to form a hydroxide that is difficult to dissolve with OH-, and aggregates on the surface of the alloy to form a dense passivation film, such as Al(OH)3, Cu(OH)2 film, etc., Effectively inhibit the polarization reaction, thereby slowing down the corrosion rate and improving the corrosion resistance of the alloy. In addition, water can also promote the formation of passivation film. It is generally believed that the following reactions are carried out during the passivation of the metal surface:
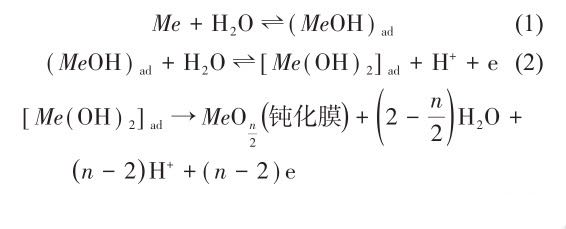
Among them, (MeOH)ad is the intermediate product, and n is the valence of the metal ion. If the solution contains negative ions A- (such as Cl-) that easily damage the passivation film, the following reactions will occur with the passivation film:
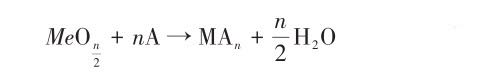
Therefore, Cl- can destroy the passivation film and produce pitting corrosion. Studies have shown that the appropriate amount of Mo can produce a self-repairing passivation film with Cr, which can effectively inhibit the pitting corrosion caused by Cl-. In addition, studies have shown that since Me-N bonding is more chemically inert than Me-Me bonding, the participation of N helps to improve the corrosion resistance of high-entropy alloy coatings.
(3) Compared with bulk high-entropy alloys, high-entropy alloy coatings can obtain a more uniform microstructure. Due to the rapid quenching effect during the preparation process, the diffusion of elements in the high-entropy alloy coating can be inhibited more effectively, thereby achieving a more uniform composition distribution and improving the corrosion resistance.
Preparation process of high-entropy alloy coating
2.1 Laser cladding technology
Laser cladding technology is a rapidly developing surface treatment method, which has the characteristics of fast cooling rate (103~106K/s) and can avoid component segregation. This technology can be used to manufacture high-entropy alloy coatings with a thickness of about 1 to 5 mm, which is much thicker than a film prepared by magnetron sputtering. Laser cladding produces a metallurgical bond between the coating and the substrate, which is stronger than the bond strength obtained by thermal spray technology. Zhang et al. used laser cladding technology to prepare FeCo-CrAlNi high-entropy alloy coatings on the surface of 304 stainless steel. The results showed that FeCoCrAlNi high-entropy alloy coatings exhibited better performance than 304 stainless steel in a 3.5% NaCl solution. Corrosion resistance and pitting corrosion resistance. Ye et al. used an electrochemical workstation to study the corrosion resistance of the laser cladding CrMnFeCoNi high-entropy alloy coating and found that the corrosion resistance of the high-entropy alloy coating is better than that of 304 stainless steel.
2.2 Magnetron sputtering technology
Magnetron sputtering technology is the most commonly used technology for preparing high-entropy alloy films. During the sputtering process, the stoichiometry of the high-entropy alloy film can be easily controlled by changing the chemical composition and process parameters of the target. Li et al. prepared FeAlCu-CrCoMn high-entropy alloy coating using magnetron sputtering technology. Electrochemical experiments showed that the corrosion resistance of FeAlCu-CrCoMn high-entropy alloy coating in 3.5%NaCl, 5%NaOH, and 10%H2SO4 solutions Better than 201 stainless steel, in addition, they also prepared FeAlCoCuNiV coating, which also has better corrosion resistance than 201 stainless steel. The corrosion performance of high-entropy alloy coatings prepared by magnetron sputtering has not been extensively studied at present, but due to the homogenization effect and satisfactory corrosion resistance of magnetron sputtering, the high-entropy The research of alloy coating will become a hot spot.
2.3 EDM deposition technology
EDM deposition is an energy-saving, material-saving, and environmentally-friendly surface treatment technology for emerging materials. It uses short pulses of high current to deposit electrode materials on the surface of the base metal. A small amount of electrode materials are melted under the action of pulsed plasma arcs, and The surface of the substrate quickly solidifies to form a coating. Li et al. prepared AlCoCrFeNi high-entropy alloy coating on AISI 1045 carbon steel by electric spark deposition. By comparing the corrosion behavior of AlCoCrFeNi high-entropy alloy cast with copper mold in 2.5% (mass fraction) NaCl solution, it was found that the coating The corrosion current of the sample is significantly lower than that of the cast AlCoCrFeNi high-entropy alloy. This is because compared to the cast AlCoCrFeNi high-entropy alloy, the Al-CoCrFeNi high-entropy alloy coating has a relatively high content of Cr oxide and Al on the surface. Oxide, and there is no Cr-rich interdendritic phase and second phase precipitation, and galvanic corrosion will not occur.
2.4 Other preparation techniques
The plasma arc cladding process has many advantages in the preparation of high-entropy alloy coatings, such as high energy exchange efficiency, small thermal distortion of parts, and low dilution of matrix materials. Cheng et al. prepared the CoCrCuFeNi high-entropy alloy coating by the plasma arc cladding process. The experiment showed that the corrosion resistance of the CoCrCuFeNi high-entropy alloy coating in 6mol/LNaCl solution was better than that of 304 stainless steel. Ge et al. prepared CuZrAl-TiNi high-entropy alloy coating on T10 substrate using mechanical alloying and vacuum hot-pressing sintering technology. Compared with T10 substrate, the corrosion resistance of CuZrAlTiNi high-entropy alloy coating in seawater solution is greatly improved. The main manifestations are high corrosion potential, wide passivation area, and secondary passivation. Niu et al. used electron beam evaporation to deposit AlxFeCoCrNiCu (x=0.25, 0.5, 1.0) high-entropy alloy coating on the alloy steel substrate mixed with the same alloying elements. The electrochemical experiment results show that Al0.5FeCoCrNiCu has high entropy The passivation zone of the alloy coating in H2SO4 and NaCl aqueous solution is greater than 700mV, and it has a higher corrosion potential (-129mV) and a smaller corrosion current density (≈2.2×10-6A/cm2). These results indicate that Al0. The corrosion resistance of the 5FeCoCrNiCu coating is better than that of the unmodified substrate.
The influence of alloying elements on the corrosion resistance of coatings
3.1 Al
Ye et al. studied the corrosion behavior of AlxFeCoCrNiCuCr(x=1,1.3,1.5,1.8) high-entropy alloy coatings in 0.05 mol/L HCl solution by the addition of Al. Electrochemical experiments showed that the addition of Al improved the coating’s corrosion behavior. Corrosion resistance, the corrosion resistance of Al x FeCoNiCrTi coating is better than that of 314 L stainless steel, of which Al 1.8 FeCoNiCuCr has the best corrosion resistance. Niu et al. studied the effect of Al on the corrosion resistance of Alx FeCoCrNiCu (x=0.25, 0.5, 1.0) high-entropy alloy coatings in a concentration of 1 mol/L H2 SO4 solution and 1 mol/L HCl solution. The study showed that In a solution with a concentration of 1 mol/LH2SO4, when the Al content is less than 0.5, it shows good corrosion resistance and pitting corrosion resistance, but when the Al content is 1.0, the corrosion resistance and pitting corrosion resistance are reduced. But still better than 304 stainless steel. In a 1 mol/L NaCl solution, the pitting resistance of Al1.0FeCoCrNi-Cu is better than that of Al0.5FeCoCrNiCu high-entropy alloy coating, and 304 stainless steel has the worst pitting resistance.
3.2 Ti
Qiu et al. studied the effect of Ti on Al2CrFeNiCoCuTix (x=0, 0.5, 1.0, 1.5, 2.0) high-entropy alloy coatings. Compared with Q235 steel, the self-corrosion current density of Al2CrFeNiCoCuTix high-entropy alloy coating is reduced by 1 to 2 orders of magnitude, and the self-corrosion potential is more "positive". With the increase of Ti content, the corrosion resistance of Al2CrFeCoCuNiTix high-entropy alloy coating in 0.5mol/LHNO3 solution improves. Shi Hai et al. prepared Ni1.5Co1.5FeCrTix high-entropy alloy coating. Research shows that with the increase of Ti content, the corrosion resistance of Ni1.5Co1.5FeCrTix high-entropy alloy coating in 0.5mol/LHNO3 solution improves. This is because the Ni1.5Co1.5FeCrTix high-entropy alloy coating surface easily forms a dense passivation film in the HNO3 solution.
3.3 Ni
Qiu et al. studied the effect of Ni content on the corrosion behavior of Al2CrFeCoCuTiNix (x=0, 0.5,, 1.0, 1.5, 2.0) high-entropy alloy coatings in 1mol/L NaOH solution and 3.5% NaCl solution. The experiment showed that with the Ni content With the increase of Al2CrFeCoCuTiNix, the corrosion resistance of Al2CrFeCoCuTiNix high-entropy alloy increases first and then decreases. Among them, Al2CrFeCoCuTiNi1.0 has the best corrosion resistance. The reason can be attributed to: Ni element has strong corrosion resistance, but its atomic radius is relatively small. When the content of Ni is high, the lattice distortion of the alloy becomes serious, which affects the microstructure of the alloy and then the alloy Corrosion resistance. Wu et al. studied the corrosion behavior of FeCoCrAlCuNix (x=0.5, 1.0, 1.5) high-entropy alloy coatings in 3.5% NaCl solution. With the addition of Ni, the corrosion resistance also showed a trend of first rising and then falling. ‐CoCrAlCuNi1.0 has the best corrosion resistance.
3.4 Mo
Li Dongliang et al. studied the influence of Mo content on the microstructure and properties of FeCrNiMnMoxB0.5 (x=0, 0.4, 0.8, 1.0) high-entropy alloy coatings. The study found that FeCrNiMn-Mo0.4B0.5 is corrosion resistant in a saturated salt cement slurry solution. The performance is the best, because Mo and Cr form a passivation film, which hinders the corrosion of Cl-. When Mo is further increased, Mo is enriched in the grain boundary, causing uneven coating composition and lower corrosion resistance.
3.5 Other elements
Cai et al. studied the effect of Cu on the corrosion resistance of FeCoCrNiCux coatings. The study showed that the addition of Cu would reduce the passivation ability of the cladding layer and make the corrosion resistance of the alloy worse. The reason can be attributed to the fact that the addition of Cu will segregate Cu in the grain boundaries to form a Cu-rich phase, which will cause galvanic corrosion, thereby reducing the corrosion resistance of the cladding layer. Cheng et al. studied the influence of Nb on the corrosion resistance of high-entropy alloy coatings. The study showed that the resistance coefficients of Nb-containing coatings are 14 times and 1.6 times that of 304 stainless steel and non-Nb coatings, respectively. This indicates that the addition of Nb element Improve the corrosion resistance of the coating. Qiu et al. studied the influence of Co content on the corrosion resistance of Al2CrFeCoxCuNiTi high-entropy alloy coatings. The study found that with the increase of Co content, the corrosion resistance of Al2CrFeCoxCuNiTi high-entropy alloy coatings in HCl and H2SO4 solutions increased. This is due to the participation of Co to form a dense passivation film on the surface of the alloy. Zhang et al. prepared FeCrNiCoBx coating by laser cladding. When 0.5<x<1.0, the corrosion resistance of the coating will increase with the increase of B content. When x is close to 1.25, the boride changes from orthorhombic (Cr, Fe) 2B to tetragonal (Fe, Cr) 2B, which will reduce the corrosion resistance of the coating, but it still shows better than ASTM304L stainless steel The corrosion resistance.
The influence of process parameters on coating corrosion resistance
Qiu et al. studied the effect of scanning rate on the corrosion resistance of laser cladding AlCrFe-CuCo high-entropy alloys. Experiments show that as the scanning rate increases, the corrosion resistance of the alloy first increases and then decreases. This is due to the rapid heating and cooling of the laser beam, the microstructure of the coating becomes fine and uniform, the segregation of components is reduced, and the corrosion resistance is improved. When the scan rate is too fast, the convection increases, the surface of the cladding layer is rough, and the corrosion resistance becomes worse.
Shon et al. studied the influence of energy input and the number of cladding layers on the corrosion behavior of laser cladding CoCrFeNi coatings. The study showed that the combination of higher energy input and double-layer cladding can reduce the dilution of the coating by the substrate, thereby It avoids the formation of local galvanic cells and shows excellent corrosion resistance in 3.5% NaCl solution.
Hsueh et al. studied the effect of substrate bias on the corrosion resistance of DC reactive magnetron sputtering (AlCrSiTiZr) N high-entropy alloy coatings. Studies have shown that a substrate bias of -100V can effectively improve the corrosion resistance of (Al-CrSiTiZr)N amorphous films, which is caused by the densification and compressive stress of the film caused by the substrate bias.
Shi Yanyan et al. studied the influence of different substrate temperatures on the corrosion resistance of magnetron sputtering FeNiCoCrMn high-entropy alloy films. The study showed that as the substrate temperature increases, the thickness of the film gradually decreases and the corrosion resistance decreases. The deposition at 100°C The film has the best corrosion resistance.
In the past 14 years, research on high-entropy alloys has opened up a huge and unexplored field of multi-component alloys. Due to its excellent properties, high-entropy alloys are expected to exert their potential in various engineering environments. This article starts from the preparation process 3, alloying elements and process parameters summarize the research progress and corrosion resistance mechanism of high-entropy alloy corrosion-resistant coatings, in order to conduct a more in-depth study on the corrosion resistance of high-entropy alloys, and promote the application of high-entropy alloys in actual industrial production , Suggestions for future research are as follows:
(1) The current research results of passivation film mainly provide microscopic characterization, and cannot explain the fundamental reason why high-entropy alloys have high corrosion resistance. Therefore, it is necessary to conduct high-resolution analysis and in-depth research on the corrosion resistance mechanism of the high-entropy alloy passivation film.
(2) The research on the microstructure of high-entropy alloys is still in the "trial and error" stage, which not only causes low efficiency, but also increases scientific research costs. Therefore, the material genome project was initiated, and the cluster structure was designed through first-principles and molecular dynamics simulations ab initio, and the equilibrium and non-equilibrium thermodynamic calculations of the high-entropy alloy coating were carried out to predict phase formation and transformation. One of the directions.
(3) A stable process system for preparing high-quality high-entropy alloy coatings has not been established. Therefore, a coating preparation process that has a uniform microstructure, reproducibility and guidance significance will be used as a corrosion-resistant high-entropy alloy coating One of the main research directions of application.